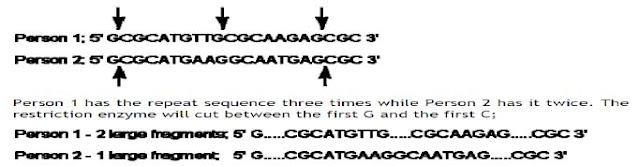
PCR-RFLP
Restriction
fragment length polymorphism (RFLP) is a technique invented in 1984 by the
English scientist Alec Jeffreys during research into hereditary diseases. It
is used for the analysis of unique patterns in DNA fragments in order to
genetically differentiate between organisms – these patterns are called
Variable Number of Tandem Repeats (VNTRs). Restriction
Fragment Length Polymorphism (RFLP) is a technique in which organisms may be
differentiated by analysis of patterns derived from cleavage of their DNA. If
two organisms differ in the distance between sites of cleavage of a particular
restriction endonuclease, the length of the
fragments produced will differ when the DNA is digested with a restriction
enzyme. The
similarity of the patterns generated can be used to differentiate species (and
even strains) from one another. Polymorphisms are inherited differences found
among the individuals in more than 1% of normal population. The
RFLP technique exploits these differences in DNA sequences to recognize and
study both intraspecies and interspecies
variation.
Principle
Restriction endonucleases are enzymes that cut lengthy DNA into short pieces. Each restriction endonuclease targets different nucleotide sequences in a DNA strand and therefore cuts at different sites. The distance between the cleavage sites of a certain restriction endonuclease differs between individuals. Hence, the length of the DNA fragments produced by a restriction endonuclease will differ across both individual organisms and species.
DNA Extraction
To begin with, DNA is extracted from blood, saliva or other
samples and purified.
PCR
Isolation of sufficient DNA for RFLP analysis is time-consuming and labor intensive.
However, PCR using specific primers can be used to amplify very small amounts of
DNA, usually in 2-3 hours, to the levels required for RFLP analysis. Therefore, more
samples can be analyzed in a shorter time.
DNA
Fragmentation/ Production of Restriction Fragments
The
purified DNA is digested using restriction endonucleases. The recognition sites
of these enzymes are generally 4 to 6 base pairs in length. The shorter the sequence recognized, the
greater the number of fragments generated from digestion.
Gel Electrophoresis
The samples
of DNA that have been treated with restriction enzymes are placed in separate lanes
on a slab of electrophoretic gel across which is placed an electric field. The fragments
migrate to wards the positive electrode, the smaller fragments moving faster than
the larger fragments, thus separating the DNA samples in to distinct bands.
Visualization
of Bands
The gel is treated with luminescent
dyes in order to make the DNA bands visible.
Applications of RFLP:
RFLPs can be used in many different settings to accomplish
different objectives.
RFLPs can be used in paternity cases or criminal cases to
determine the source of a DNA sample. (i.e. it has forensic applications). RFLPs
can be used determine the disease status of an individual. (e.g. it can be used
in the detection of mutations particularly known muations). RFLPs can be used
to measure recombination rates which can lead to a genetic map with the
distance between RFLP loci measured in centiMorgans.